News

Dr Gloria Gonzalez-Aseguinolaza, Chief Scientific Officer of Vivet Therapeutics Receives the Rosalind Franklin Society Special Award in Science
Paris, France, July 16, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic
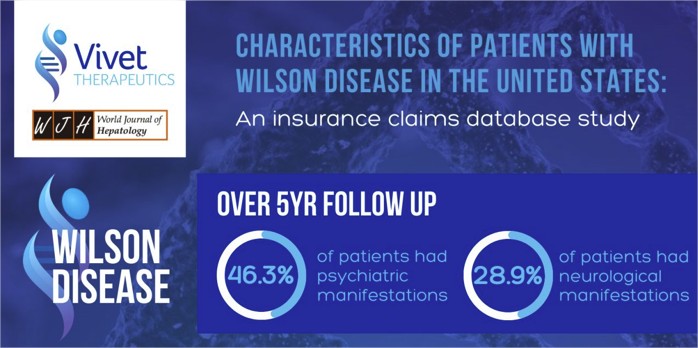
Characteristics of patients with Wilson Disease in the United States: An insurance claims database study
We are delighted to share our findings in a peer reviewed paper published in the World Journal of Hepatology, titled ‘Characteristics of patients with Wilson

Vivet Therapeutics presents interim data on its Phase 1/2 GATEWAY trial for the Treatment of Wilson Disease at EASL Congress 2024
Vivet Therapeutics Presents Interim Data from its Phase 1/2 GATEWAY Trial for the Treatment of Wilson Disease at EASL Congress 2024 VTX-801 increased ceruloplasmin ferroxidase
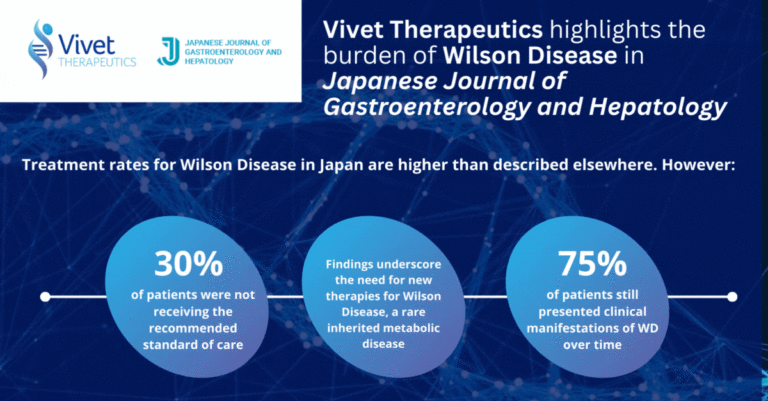
Study on Treatment of Wilson Disease in Japan Published in Japanese Journal of Gastroenterology and Hepatology
Paris, France, May 30, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic
Vivet Therapeutics Doses First Patient in Cohort 2 in Phase 1/2 GATEWAY Clinical Trial for Treatment of Wilson Disease
Vivet Therapeutics Doses First Patient in Cohort 2 in Phase 1/2 GATEWAY Clinical Trial for Treatment of Wilson Disease

Rare Disease Day – February 29
To mark #RareDiseaseDay on 29 February, our CEO Jean-Philippe Combal, spoke to pharmaphorum

Vivet Therapeutics receives EUR 4.9 million to advance development of a gene therapy
PRESS RELEASE Vivet Therapeutics receives EUR 4.9 million to advance development of a gene therapy for the treatment of cerebrotendinous xanthomatosis

Vivet Therapeutics Announces Presentations at Upcoming European Society of Gene and Cell Therapy (ESGCT) 2023 Annual Congress
Paris, France, October 23, 2023 (GLOBE NEWSWIRE) – Vivet Therapeutics (“Vivet”), a clinical- stage biotechnology company

Vivet Therapeutics awarded with the Prix Galien MedStartup in New York
Vivet Therapeutics is very pleased and proud to have won the Prix Galien MedStartup Award for “Best Collaboration For the Developing Or Underserved Populations Worldwide”.

GATEWAY clinical trial for Wilson Disease has now its website
Wilson Disease is a rare, progressive genetic disorder that causes excess copper to be stored in the body.

VTX‐801 Receives U.S. FDA Fast Track Designation for the Treatment of Wilson Disease
VTX-801 Receives U.S. FDA Fast Track Designation for the Treatment of Wilson Disease

Gene Therapy aims to be a one-time treatment that may stop or slow the progression of Wilson disease
Learn how gene therapy aims to target the cause of Wilson disease by delivering a working ATP7B gene into cells. ClinicalTrials are now open for this investigational therapy.

Congratulations to all Women in Gene Therapy and to Dr. Gloria González-Aseguinolaza our CSO @Vivet Therapeutics!
Congratulations to all Women in Gene Therapy and to Dr. Gloria González-Aseguinolaza our CSO @Vivet Therapeutics!

Mirum Pharmaceuticals and Vivet Therapeutics Enter into Exclusive Worldwide Option and License Agreement for Vivet’s PFIC Gene Therapy Programs
Mirum Pharmaceuticals and Vivet Therapeutics Enter into Exclusive Worldwide Option and License Agreement for Vivet’s Gene Therapy Programs Targeting Progressive Familial Intrahepatic Cholestasis

Vivet and Pfizer Inc. Announce FDA Authorization to Proceed with GATEWAY, the Phase 1/2 Study for VTX-801, Vivet’s Investigational Gene Therapy for WD
Vivet Therapeutics and Pfizer Inc. Announce FDA Authorization to Proceed with GATEWAY, the Phase 1/2 Study for VTX-801, Vivet’s Investigational Gene Therapy for Wilson Disease

Vivet Therapeutics and Pfizer Inc. Enter into Manufacturing Agreement for Vivet’s Investigational Gene Therapy for Wilson Disease
Vivet Therapeutics and Pfizer Inc. Enter into Manufacturing Agreement for Vivet’s Investigational Gene Therapy for Wilson Disease
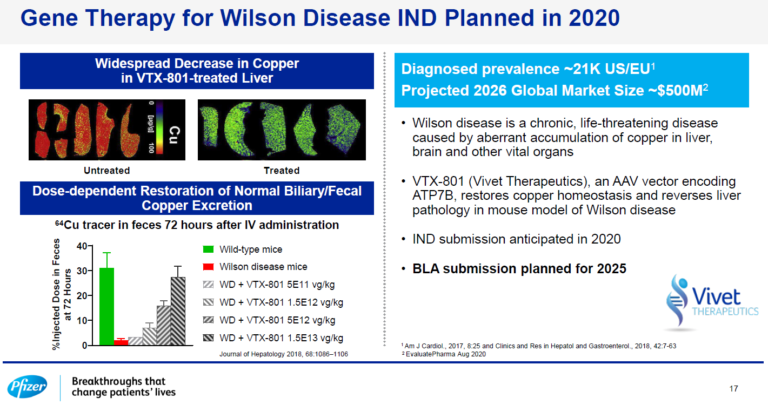
Vivet’s lead program, VTX-801 for Wilson Disease, highlighted during Pfizer’s Investor Day September 15th 2020
Vivet Therapeutics’ lead program, VTX-801 for Wilson Disease, was highlighted during Pfizer’s Investor Day September 15th 2020.

Vivet’s Second Gene Therapy Product, VTX-803 for PFIC3, Receives US and European Orphan Drug Designation.
PARIS, France June 1st, 2020, Vivet Therapeutics announced today that both the Food and Drug Administration

Happy to share the March 2020 Info Wilson newsletter from ABPWilson!
Vivet is very happy to share the March 2020

Vivet Announces Publication in Nature Communications of VTX-803 Preclinical Data
Vivet Announces Publication in Nature Communications of Preclinical Data from VTX-803

