Our DNA
Building Transformational Therapy
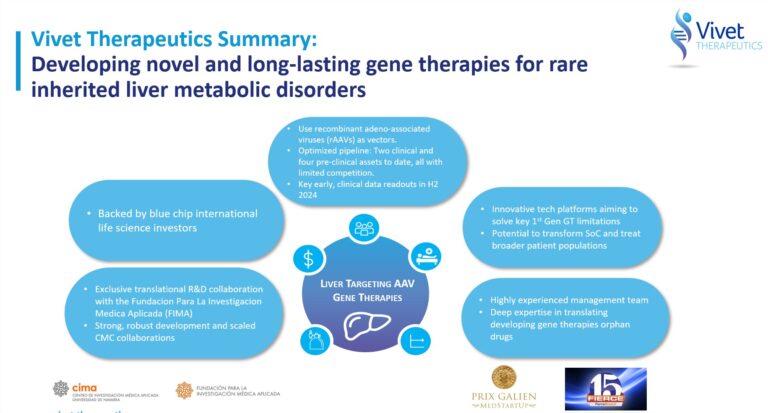
Vivet Therapeutics is a clinical-stage emerging biotechnology company developing novel gene therapy treatments for rare, inherited metabolic diseases.
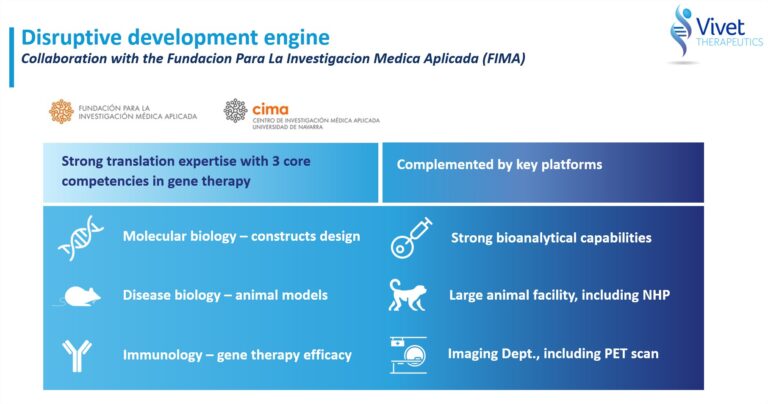
Vivet is building a diversified gene therapy pipeline based on novel recombinant adeno-associated virus (rAAV) technologies developed through its partnerships with, and exclusive licenses from, the Fundación para la Investigación Médica Aplicada (FIMA), a not-for-profit foundation at the Centro de Investigación Medica Aplicada (CIMA), University of Navarra based in Pamplona, Spain.
Vivet’s lead program, VTX-801, is currently under clinical development (GATEWAY – Phase 1/2 Clinical Trial of VTX-801 in Wilson Disease).
VTX-801 is a novel investigational gene therapy for Wilson Disease, which has been granted Orphan Drug Designation (ODD) by the Food and Drug Administration (FDA) and the European Commission (EC) and Fast Track designation by the FDA. Wilson Disease, a rare genetic disorder, is caused by mutations in the gene encoding the ATP7B protein, which reduces the ability of the liver and other tissues to regulate copper levels, causing severe hepatic damage, neurologic symptoms and potentially death.
VTX-801 is a novel, investigational rAAV-based gene therapy vector designed to deliver a miniaturized ATP7B transgene encoding, a functional protein that has been shown to restore copper homeostasis, reverse liver pathology and reduce copper accumulation in the brain of a mouse model of Wilson Disease. VTX-801’s rAAV serotype was selected based on its demonstrated tropism for transducing human liver cells.
Vivet’s second gene therapy product, VTX-803 for PFIC3, received US and European Orphan Drug Designation in May 2020.
Vivet is also working on technological platforms addressing key challenges of gene therapy, including sustained therapeutic gene expression in young patients, adults and immune responses towards the viral vector.
Vivet is supported by international life science investors including Novartis Venture Fund, Roche Venture Fund, HealthCap, Pfizer Inc., Columbus Venture Partners, Ysios Capital, Kurma Partners and Eurazeo.